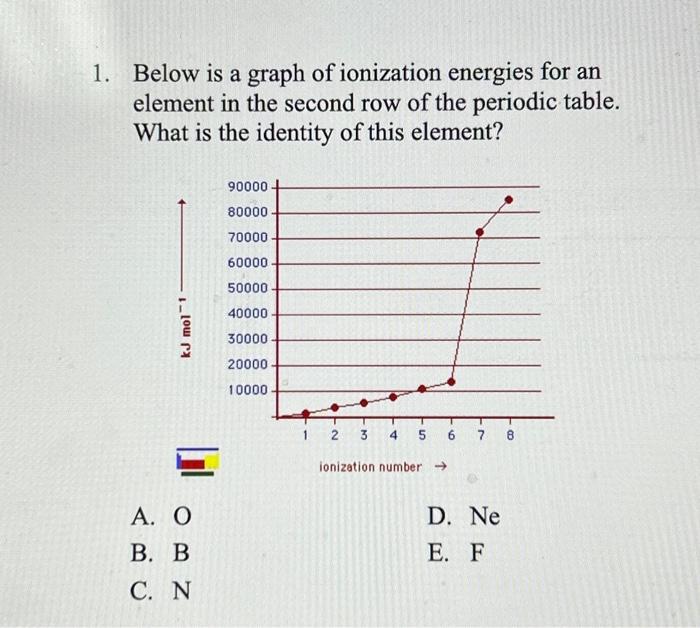
Solved 1. Below is a graph of ionization energies for an Chegg
Ionization energy across a outlet row on the periodic table
Share. Visit »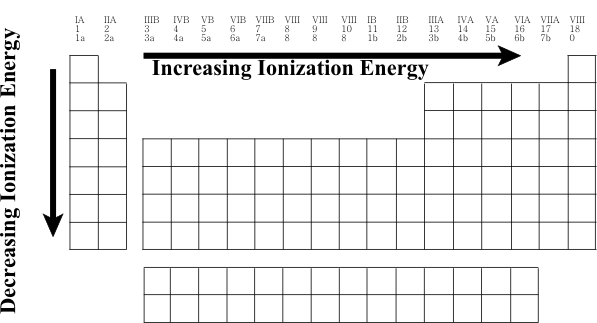
Periodic Table
Periodicity Definition in Chemistry
What is the trend of ionization energy within groups and across
How to Find Ionization Energy EnthuZiastic
What Is Ionization Energy Definition and Trend of the periodic table the ionization energy increases.jpg)
What is the electron configuration of neutral phosphorous ppt
What is the trend of ionization energy within groups and across
Periodic Trends in Ionization Energy:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Easy To Use Chart of Periodic Table Trends
Periodic Trends in Ionization Energy Chemistry Socratic
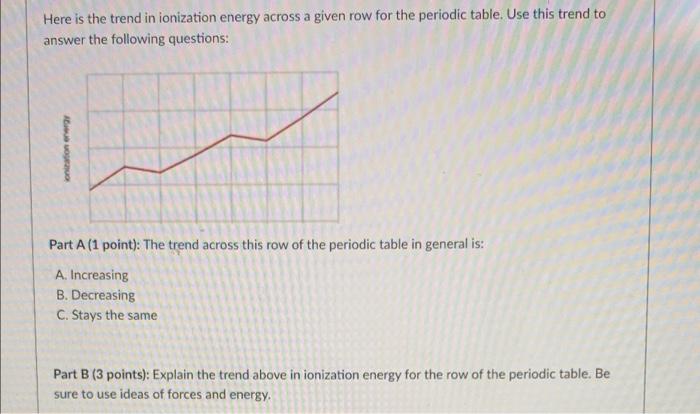
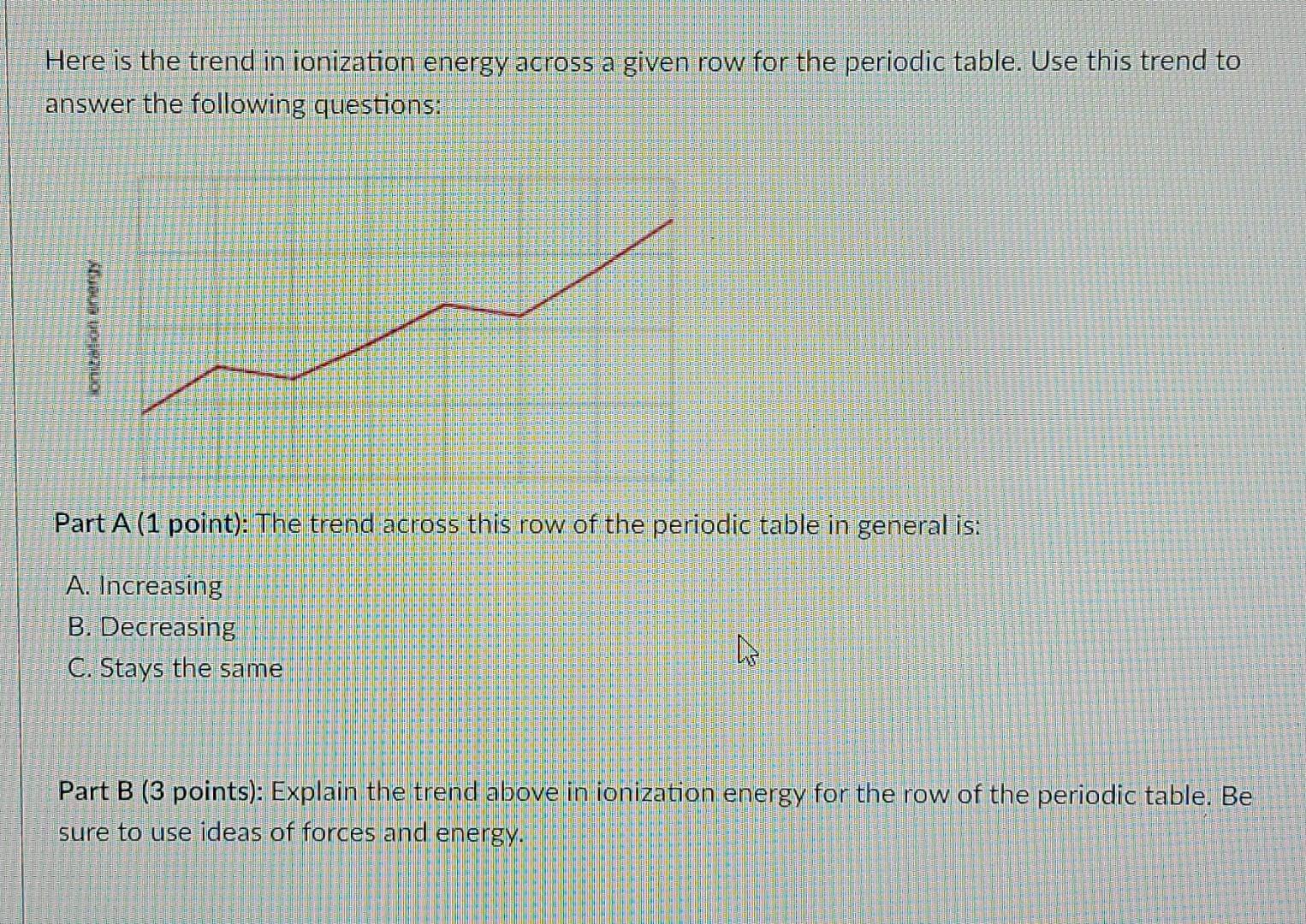
Solved Here is the trend in ionization energy across a given
Solved Here is the trend in ionization energy across a given
Ionization energy Wikipedia
What is the trend of ionization energy within groups and across
4.4 Ionization energy and Electron Affinity Chemistry Fundamentals
6.6 Ionization Energies Chemistry LibreTexts
Atomic Ionic Radius Trend Definition Differences Chart Video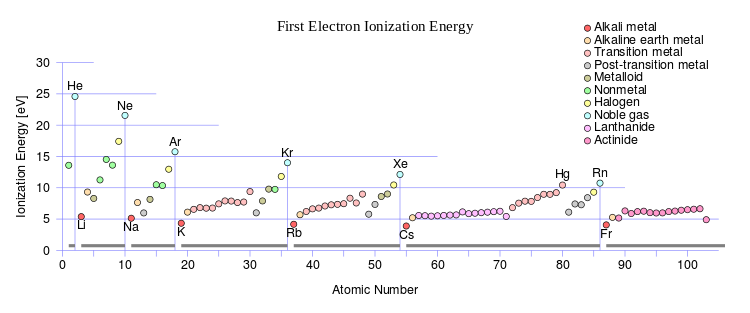
How does ionization energy change across a period and down a group
2. Periodic Trends
First And Second Ionization Energy The Periodic Table Variations
Periodic table Wikipedia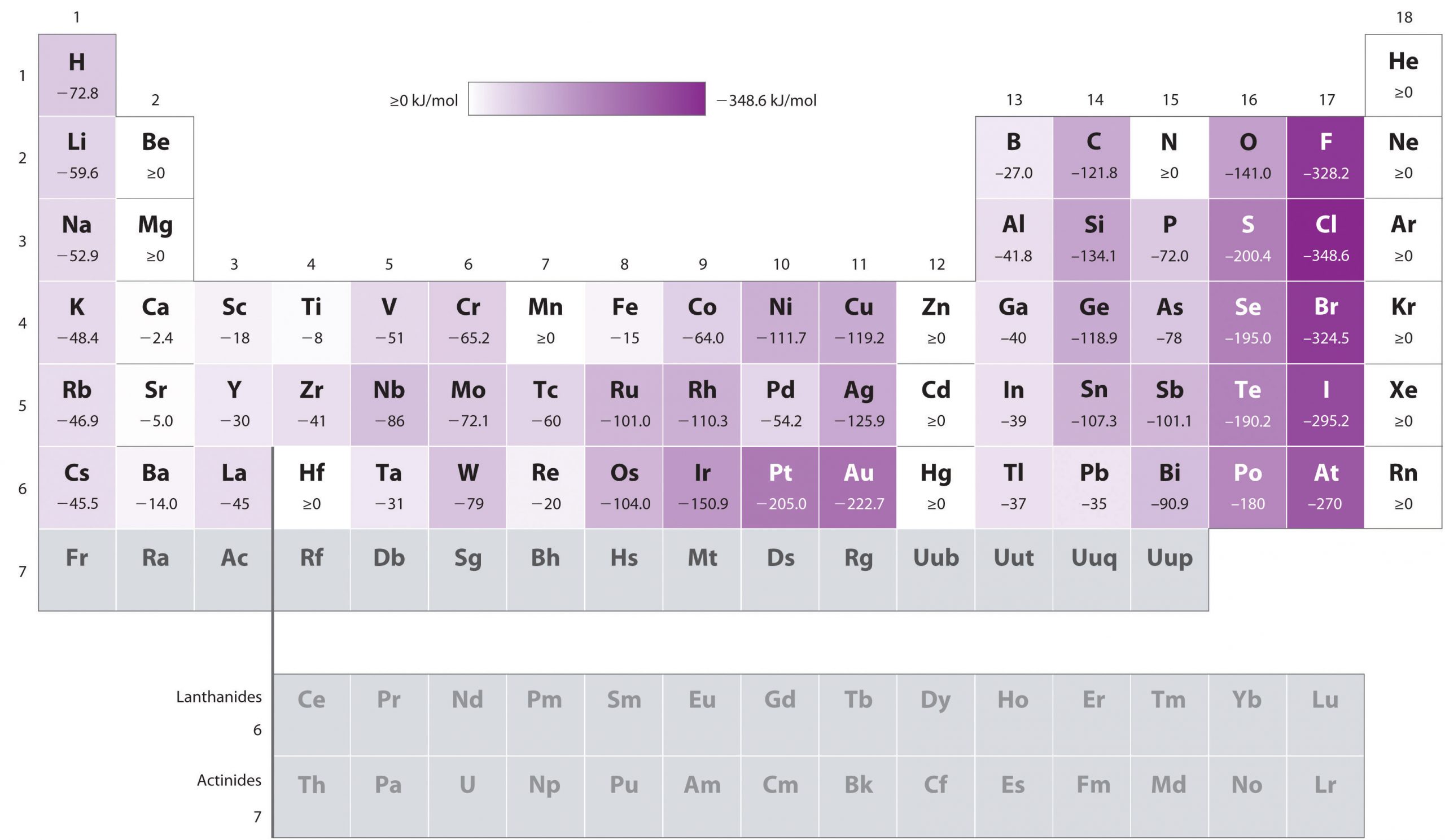
4.4 Ionization energy and Electron Affinity Chemistry Fundamentals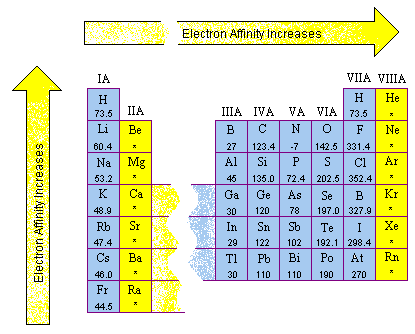
Ionization Energy and Electron Affinity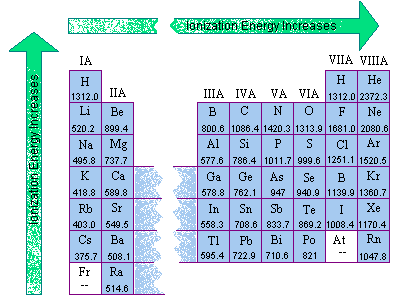
Ionization Energy and Electron Affinity
How do you explain the trend of ionization energies as you go from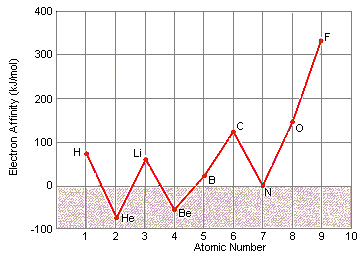
Ionization Energy and Electron Affinity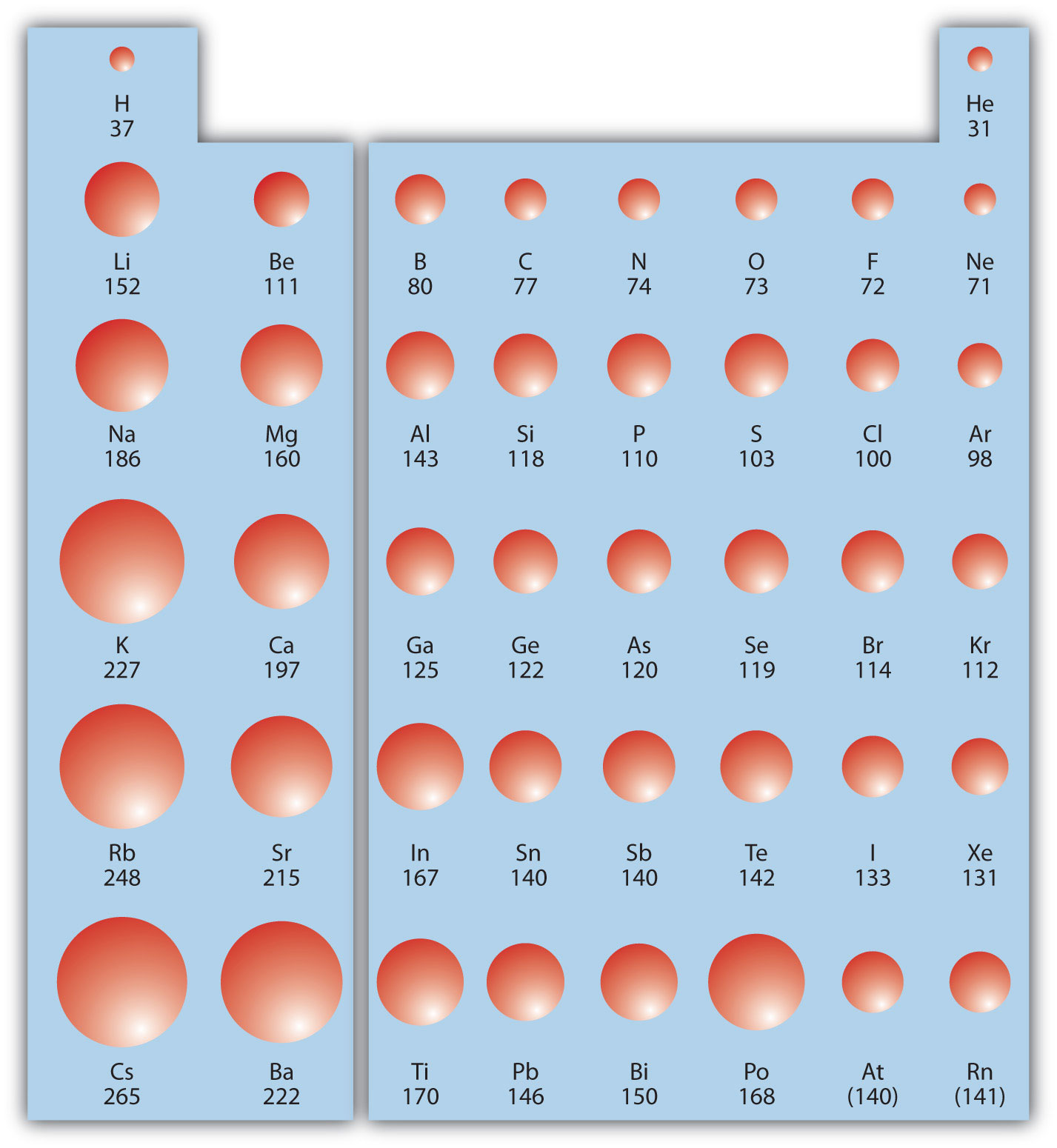
Periodic Trends
Solved Here is a table of the first ionization energies Chegg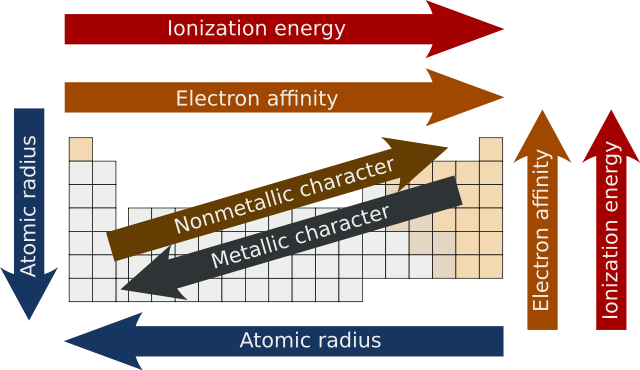
Periodic trends Wikipedia
Can someone explain why A is wrong and B is right. I thought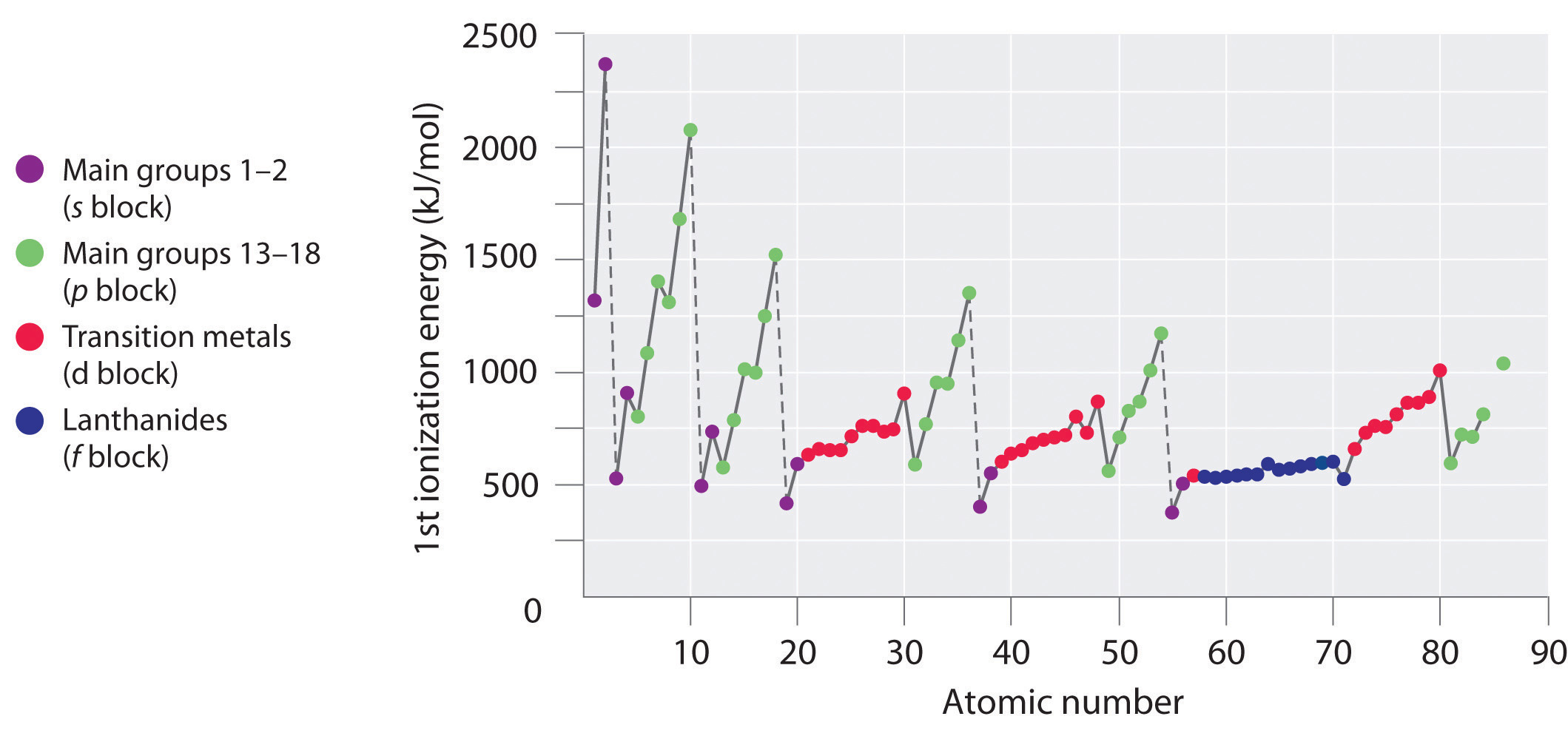
The Periodic Table and Periodic Trends
What are the periodic trends for atomic radii ionization energy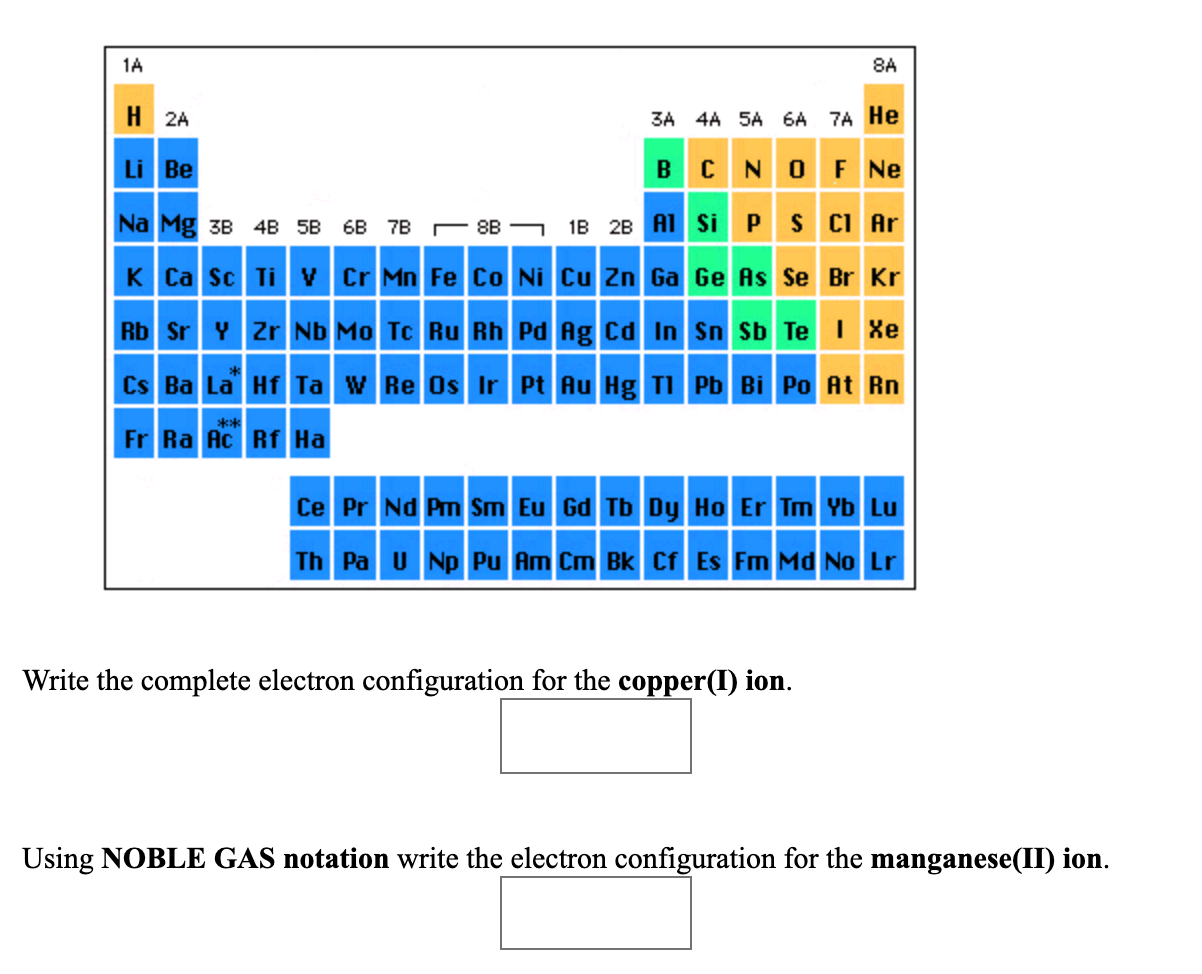
Solved In general in which direction on the periodic table
What are some patterns that appear on the periodic table Quora
Periodic Trends Chemistry LibreTexts
Lab on graphing and analyzing Ionisation energies by Jason Liu
ANSWERED Going down a group in the periodic table atomic size
Ionization Energy Definition Trends Factors Lesson Study
S3.1.3 How do 1st ionisation energies change across period 2 and 3 elements