
Chemical bonding Atomic Structure Intermolecular Forces
Element in a row have the same outlet orbital electrons foces
Share. Visit »
Orbital Shapes EWT
Electron Configuration Overview Levels Patterns Video
Orbital Shapes EWT
What determines the atom size If I have two atoms with the same
How to know if an element has a different ground state electron
PDF Atomic Orbitals Explained with Classical Mechanics
Chemical bonding Atomic Orbitals Shapes Hybridization Britannica
Chemical bonding Atomic Orbitals Shapes Hybridization Britannica
Electron Configuration an overview ScienceDirect Topics
Atomic Ionic Radius Trend Definition Differences Chart Video
Electron Configurations
Periodic table Wikipedia
What do atoms in the same period have in common Quora_-_electron_orbitals.svg/800px-ADOMAH_periodic_table_(horizontal)_-_electron_orbitals.svg.png)
Core electron Wikipedia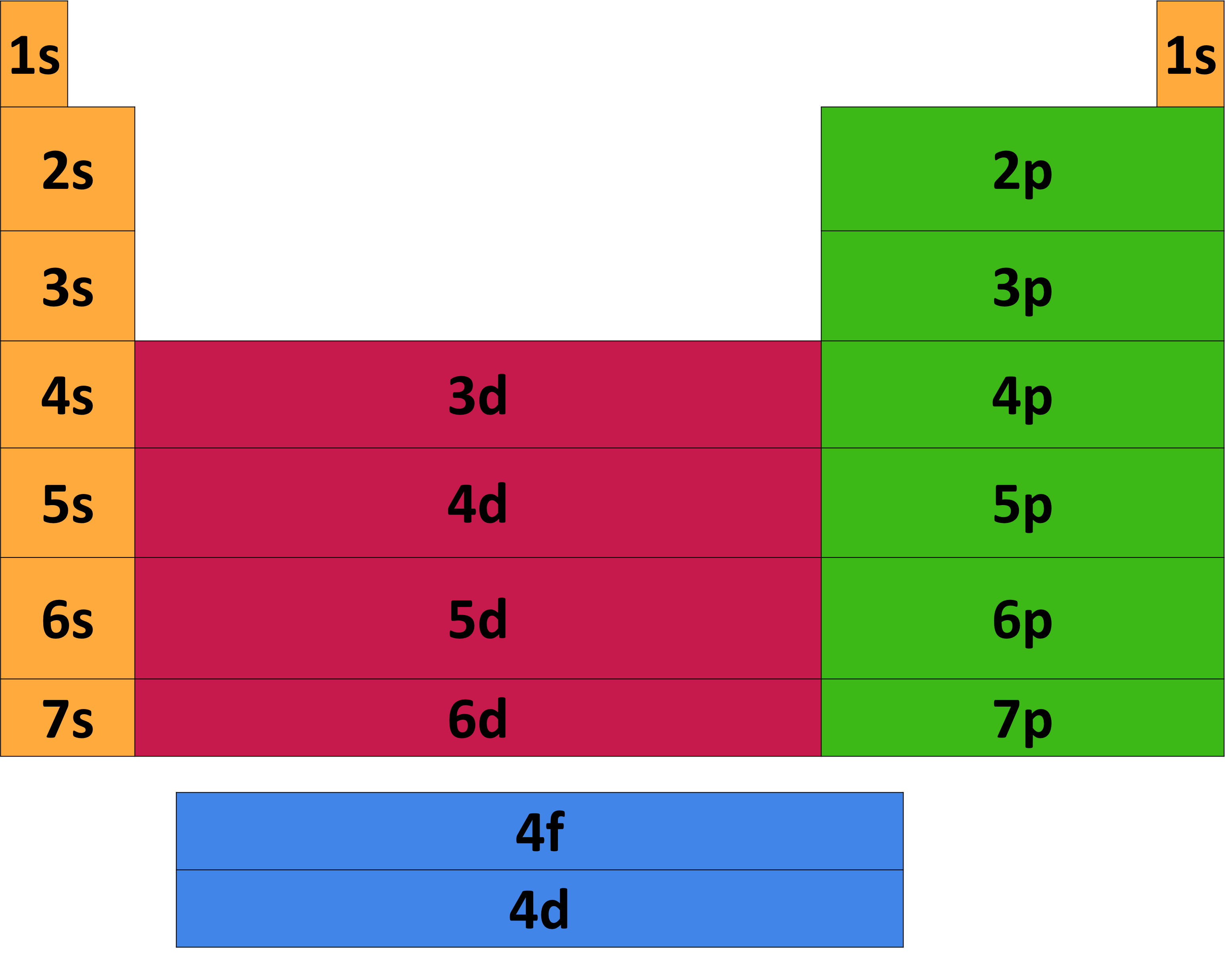
Electron Configuration Overview Examples Expii
Organizing Atoms and Electrons The Periodic Table Annenberg Learner
Electron Configuration Overview Levels Patterns Lesson
Solved reactions. For second row elements these are the 2s Chegg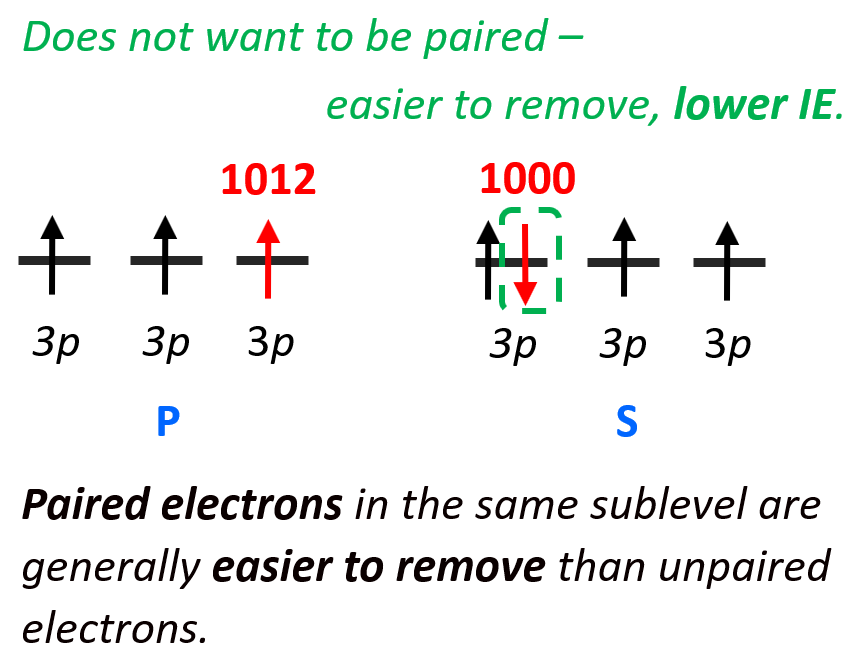
Ionization energy Chemistry Steps
Orbital Shapes EWT
Aufbau Principle ChemTalk
Why do atoms within the periodic table differ in electronegativity
1.3 Day 3 Orbital Energy and Electron Configuration Chemistry
Electron Configurations First Three Rows Stone Cold Chemistry Talk:max_bytes(150000):strip_icc()/energylevels-56a129545f9b58b7d0bc9f39-5aeb7f1aae9ab800373981a3.png)
Electronic Structure and the Aufbau Principle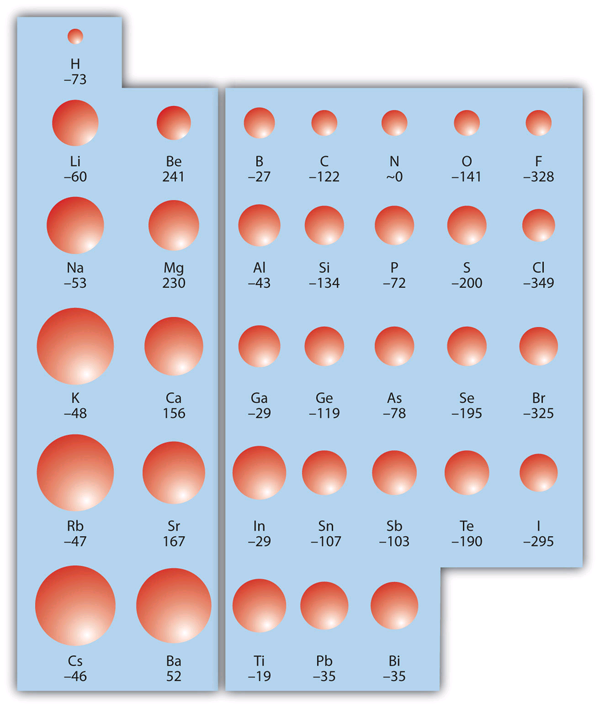
Periodic Trends Introductory Chemistry 1st Canadian Edition
Quantum Numbers and Electron Configurations
Chemical bonding Atomic Orbitals Shapes Hybridization Britannica
Atomic orbital Wikipedia
Why does iron have 14 electrons in its 3 orbit Can inner shells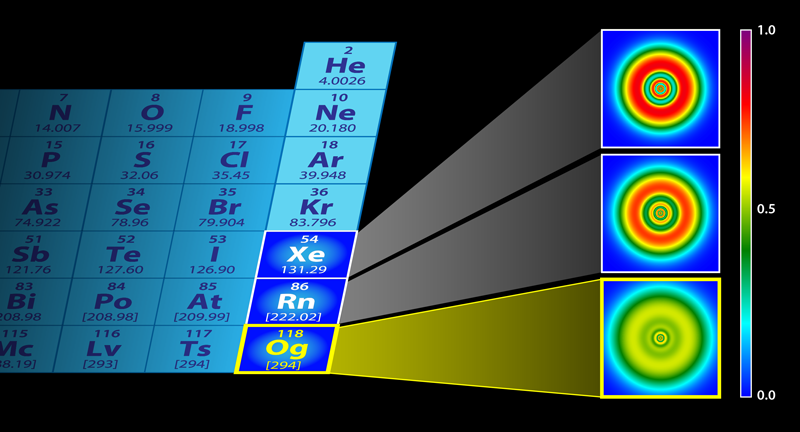
Physics Heaviest Element Has Unusual Shell Structure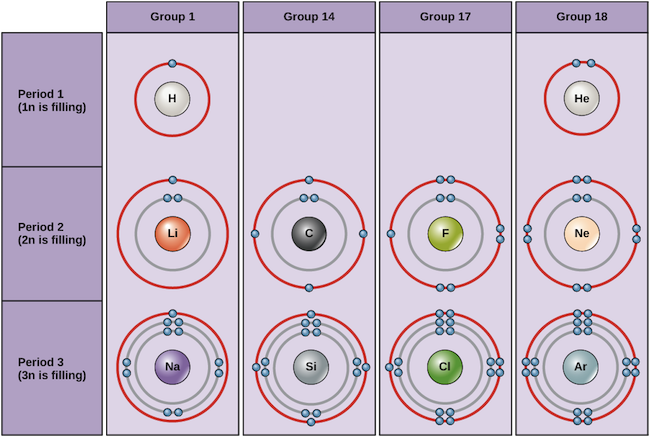
The periodic table electron shells and orbitals article Khan
What do atoms in the same period have in common Quora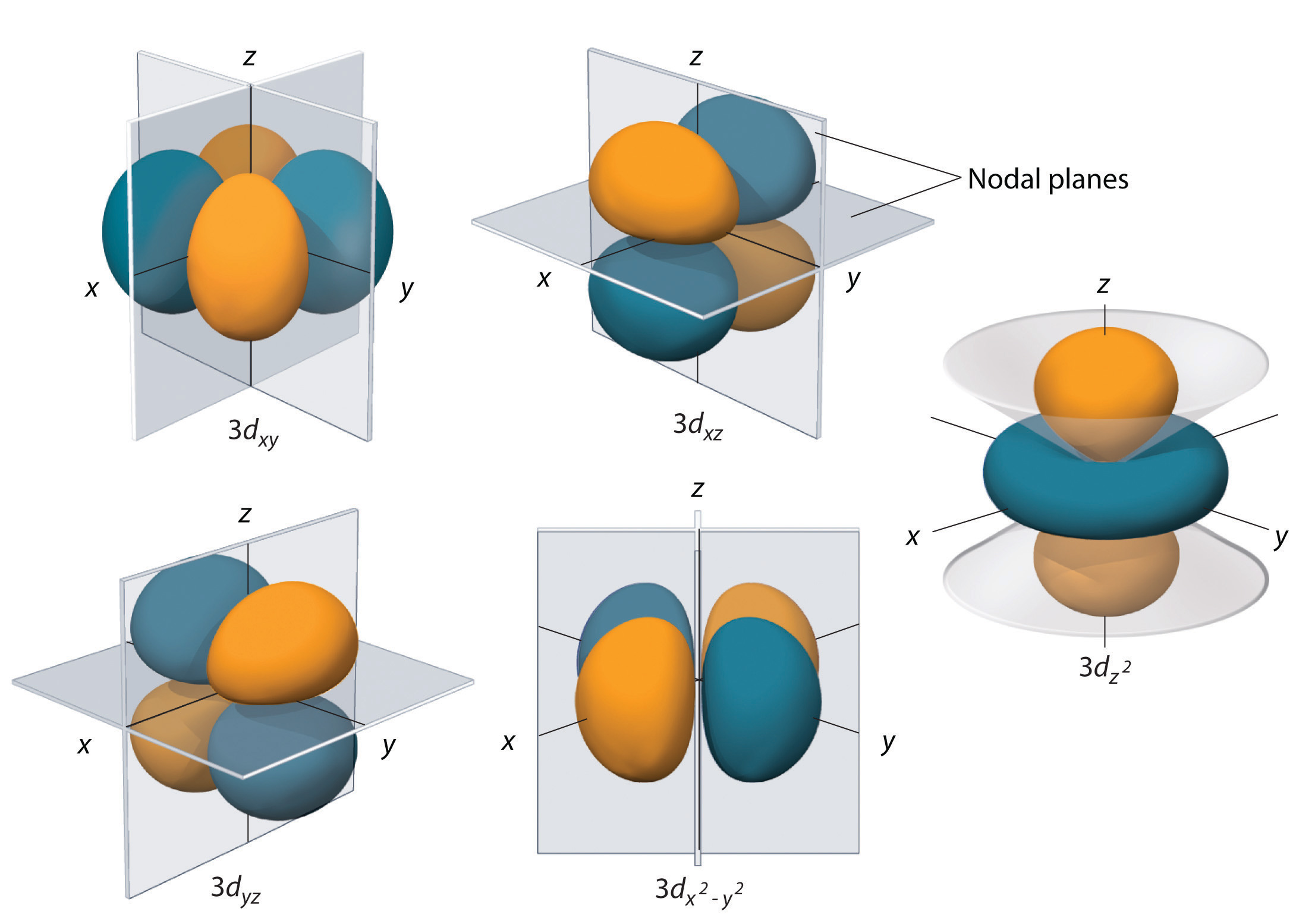
Chapter 2.5 Atomic Orbitals and Their Energies Chemistry LibreTexts
Electron configuration Wikipedia
Lewis Structures Overview Structural Formula Examples
Understanding the Uniqueness of 2p Elements in Periodic Tables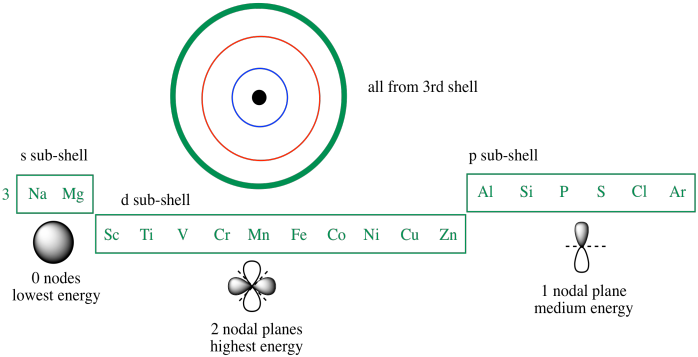
Structure Reactivity Atoms
Chemistry Which pair of elements would be expected to have the